
TAY-R1: First-in-Class Oral Small Molecule Gene Therapy
TAY-R1 is a first-in-class, orally available small molecule designed to treat Recessive Dystrophic Epidermolysis Bullosa (RDEB), a rare and often fatal genetic disease. TAY-R1 restores therapeutic levels of collagen VII in RDEB patient fibroblasts by overcoming nonsense mutations, offering potential as a disease-modifying treatment.
Tay is actively seeking licensing and commercial partnership opportunities for TAY-R1, which may also be applicable to hundreds of additional genetic conditions, providing substantial indication expansion potential.
TAY-B1 (Repibresib): Locally Active BET Inhibitor
TAY-B1 is a first-in-class, locally active BET bromodomain inhibitor (BRD2, BRD3, BRD4, BRDT) designed to effectively target inflammatory and fibrotic pathways while minimizing systemic exposure. As a soft drug, TAY-B1 is engineered for localized efficacy with an excellent safety profile, addressing key limitations of systemic BET inhibitors.
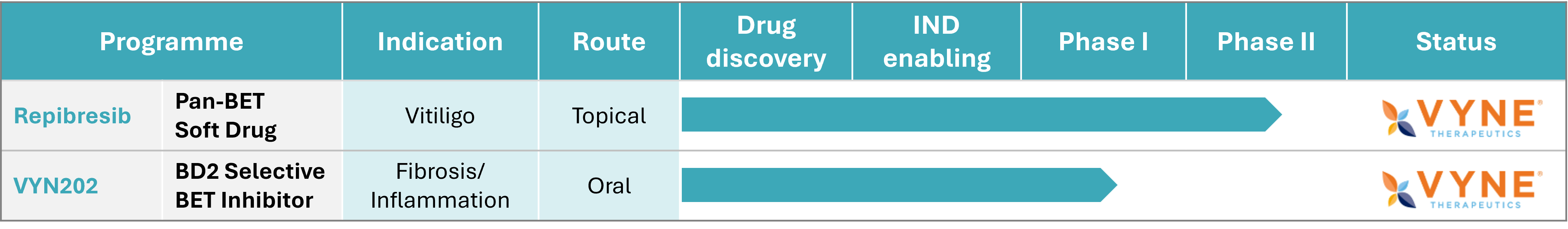
Successfully licensed to VYNE Therapeutics in 2021, TAY-B1 has progressed to a Phase 2b clinical trial in vitiligo, with proof of concept demonstrated in a Phase 1b study after just 16 weeks of treatment.
Beyond vitiligo, TAY-B1 has shown compelling preclinical efficacy in psoriasis and wound healing models, underscoring its potential for broader dermatological and fibrotic indications.
TAY-B2: Oral, BD2-Selective BET Inhibitor
TAY-B2 is a next-generation, orally bioavailable BET inhibitor with high selectivity for bromodomain 2 (BD2), designed to address systemic inflammatory and fibrotic diseases where broad tissue penetration is essential. TAY-B2 delivers potent anti-inflammatory and anti-fibrotic activity, demonstrating picomolar efficacy against key inflammatory cytokines in cellular assays and robust preclinical efficacy in models of inflammation and organ fibrosis.
TAY-B2 (designated VYN202 by VYNE Therapeutics) was successfully licensed to VYNE in 2023 and has advanced into a Phase 1b clinical trial, supporting its strong commercial and clinical potential.
At Tay Therapeutics, our partnering strategy focuses on collaborating with companies that have the capabilities and resources to advance our assets through clinical proof-of-concept (PoC) milestones. We seek partners who share our values and commitment to delivering transformative therapies to patients in areas of high unmet medical need.
This approach is exemplified by our successful Option and License Agreement with VYNE Therapeutics, signed in April 2021, followed by the exercise of options for both TAY-B1 and TAY-B2. VYNE, a NASDAQ-listed biotech developing innovative therapies for immune-inflammatory disorders, has delivered significant revenue to Tay through this partnership. To date, Tay has secured over £6 million in research funding, option fees, upfront payments, and development milestones.
The TAY-B1 license was exercised in August 2021, enabling the development of a topical formulation, now known as Repibresib (VYN201), which has progressed into a Phase 2b clinical trial in vitiligo.
The TAY-B2 license (designated VYN202 by VYNE) was exercised on April 28, 2023, and has since advanced into a Phase 1b clinical study.
Tay is actively seeking licensing and commercial partnership opportunities for its Tay-Enable™ asset, TAY-R1.